|
all figures |
|||||||||||||||||||||||
|
|
|||||||||||||||||||||||
 |
|
Trace analysis by Micro-Planar-Chromatography |
|
This chapter needs so much additional experimental and error controlling work, that it can be written only in parts over the next months. Therefore by now here PART ONE: |
|
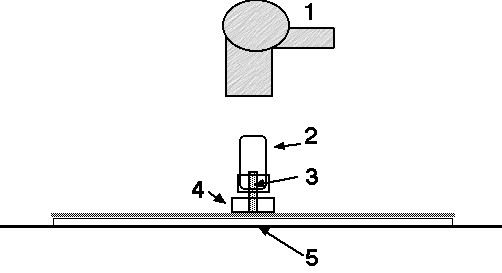
Concept and technical details: * We use the µ-PLC technique published in this Open Access book. We add a circular sample bottle holder and a strong cold air blowing hair dryer. We need an especially permeable wick in the 5 mm o.d. PTFE tube. The tube is together with a PTFE sealing ring a compact part for top hardware purity conditions, see figure 1. Thus the sample is not in contact with the screw top. A one ml water sample is transferred by a clean pipette into a clean 1.5 ml micro bottle. The bottle is closed with the above mentioned compact PTFE sealing disk compactly connected with the 5 mm o.d. sample - and mobile phase transfer tube plus wick. A figure of the PTFE sealing ring will be shown at the end of this part 1 soon. |
|
|
|
* The water sample flows from a 1.5 ml micro flask through the wick accurately into the center of a top pre cleaned HPTLC plate of 100 x 100 mm size at room temperature. * Now the sample bottle is filled with one ml freshly distilled cleanest methanol. Under still flowing dryer air the bottle is reinserted into the center PTFE holder ring. Because of the lower viscosity the methanol flow is now larger than 50 µl/min, thus the residual sample parts and all remaining traces in the transfer wick are quicker transferred into the circular area of the adsorbed water impurity substances. The transmitting methanol dries off sharply outside the PTFE bottle holder ring, focussing possible substances from the water sample. * Depending on the sample impurity level we can transfer instead of a 100% water sample mixtures of water with volatile solvents like methanol, ethanol, butanol, acetone, diethyl ether. This lowers of course the best possible detection / quantitation level but may avoid chemisorption of impurities on glass and/or the wick material. * The next steps are standard µ-PLC procedures checking BUT if necessary or advisable only. However we may use such “No Drinking Water” chromatogram data to support clean-up-techniques and installations. In this case it may be necessary to identify parts of the signals. They may correlate with repairable problems in the clean-up process and thus help to identify error sources like not yet correctly cleaned pumps or other hardware parts. If the drinking water data found by the water-µ-PLC concept given here show ZERO substance signals we however need further effort, because we cannot state, the water is OK. > The water is possibly not of drinking quality because of bio impurities Quantitation limits: Detectability limits: At the end of this “part 1” - µ-PLC trace analysis : |
|||
|
|
|||
 |
|||
 |
|||
|
|
|
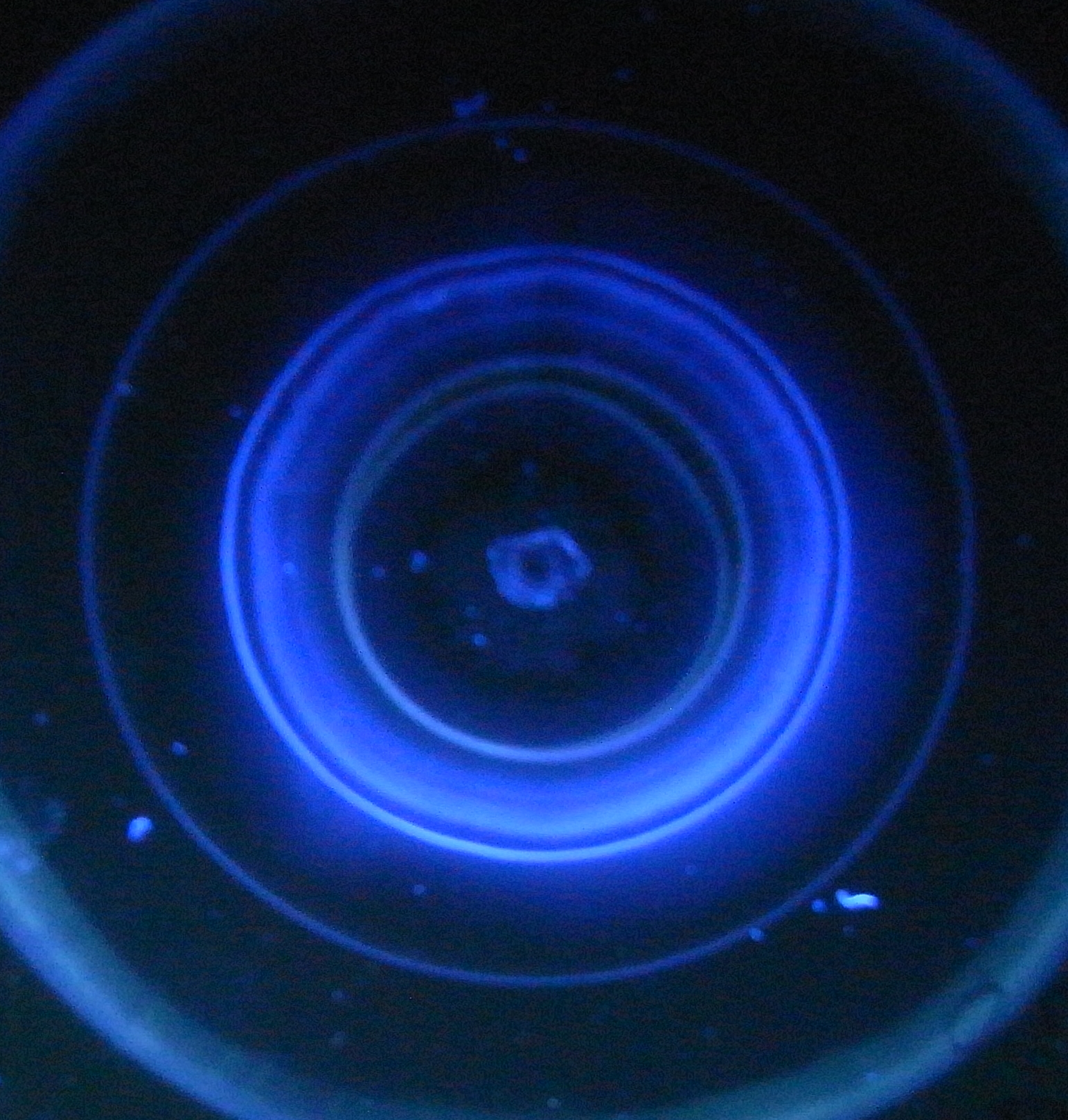
Sampling of a mix of 0.5 ml clean methanol and 0,5 ml water sample needs about 29 minutes time under constant hair dryer action. When all sample entered the plate layer the bottle and its holder are removed. The still wet central part of the sample area is dried completely with the air blower still on. It blows all the time a strong enough air stream at 20 degree centigrade onto the plate. When dry the plate is covered with the standard cover glass plate - see figure 4. The air blower is switched off now. The sample area is focussed with - normally - freshly distilled methanol as mentioned above and again dried with the cover glass plate taken off. Figure 5 shows a very first result for a (quite) clean water sample under fluorescence. |
|
|
||||||||||||||||||||||||
|
Analytical details to rain-, snow-, tap-, fresh and very old bottle-water, ground-, surface-, river-water will follow. There have been found new possibilities to change the selectivity. |
|
PART TWO: First experiments with fruit liquids, as an example wine, look promising. We learned, that drying steps in between are necessarily to be done until total uniformity of the whole plate. The two figures below show drying errors resulting in separation selectivity effects - see the circle deformation. Sample amount into the plate center was 0.50 ml, drying time to get rid of the more than 85 % water in the wine was only 30 minutes. The enriched wine constituents were concentrated on a sharp circle as similar to figure 5 above. Lots of detailed studies however are necessarily to be done.
|
|||||
 |
|||||
 |
|||||
|
scroll bar --> |