|
From Multi Integration to the “preliminary” or the final Quantitation
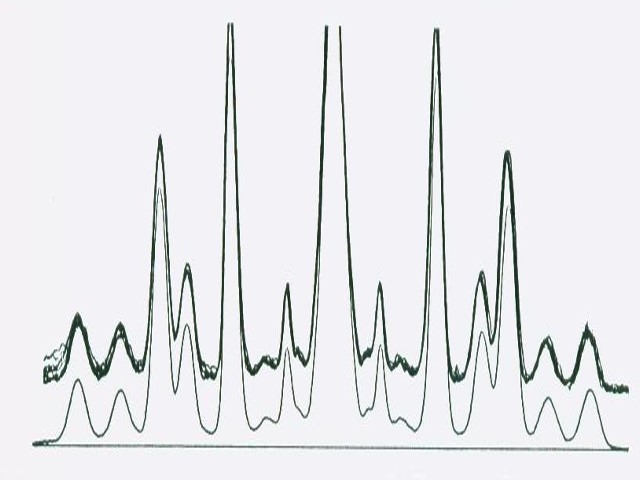
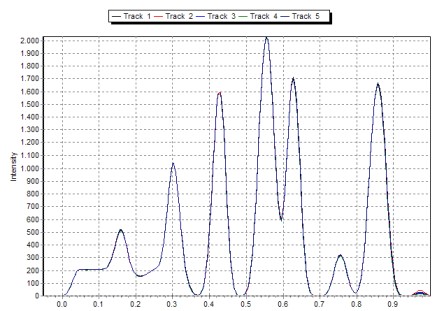
The integrated peak area is only part of the substance circle or the substance bow. It is the larger the broader the photo scan track.
Let us first discuss the situation with a single substance full circle chromatogram.
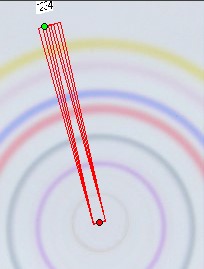
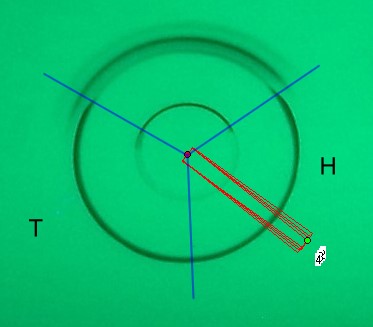
The length of the substance circle depends on its radius “r”. The circle length “L” equals
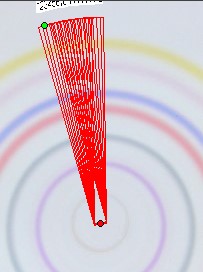
3.1415 * 2 * r [mm]. The width of the photo scan track is “b” [mm]. This is only a small part of the full circle length “L”. Therefore this integral value correlates only with a small part of the total substance. As circular µ-PLC on qualified HPTLC plates show precise circles if correct done we can calculate the total substance integral value “Vi” for the substance “i” . We need the substance position in the chromatogram and the integral data based on the scan track width “b” .
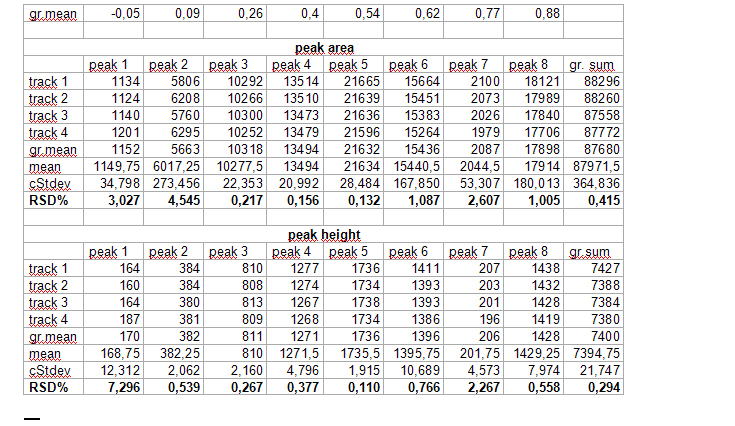
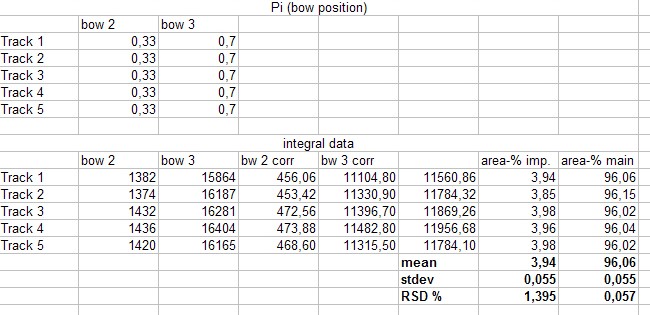
We do not take the radius of the substance circle in [mm] but its relative value = “Pr” This value follows from the substance circle radius = “Pi” divided by the mobile phase front circle radius = “Pf” which is Pr = Pi / Pf . It is NOT necessary to measure both radius values as the relative position Pr is automatically calculated by the Multi Integration video densitometer software. By now in Version 2.0 (2009) this Pr value is named “Rf” but in this book the use of (circular) Rf values is refused as useless in practice of circular µ-PLC. Reasons: The chromatogram starts not in the plate center but from the focussed start circle and the position of the mobile front circle changes in multi phase separations drastically from run to run. Therefore there is nothing like a true Rf-value. The relative substance position value however is very precise at the moment the quantitation photo is taken and it is printed in the multi integration report - see the data table at the end of this chapter. “Pr” is the key value to correct all track width based integral values into full circle integral values. Only these data allow to express preliminary quantitative results. The procedure is very simple: Let us name the track width based peak integral of substance “i” as “Mi” and the extrapolated peak integral of the total substance amount as “Vi” and “b” the width of the integration track.
It holds that Mi/b = Vi/(Pi * 2 * 3.1415) or Vi = Mi * Pi * 2 * 3.1415/b
Thus Vi = Mi * Pi, because all quantitation is based on relative peak area values and
b / 2 * 3.1415 are equal and constant for all peak integrals. They disappear in preliminary quantitation calculations. Instead using Pi (the radius) we can use the Pr value for the integral extrapolation. Pr is available on screen or in print , Pi not.
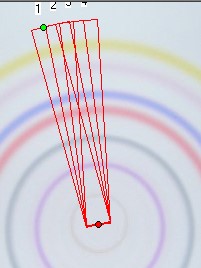
This extrapolation is also valid for multi sample bow chromatograms. We simply multiply all integral data of all separated substances by the corresponding Pr values for each substance. These data are given by the quantitation report of the SORBFIL videodensitometer software - see the example below.
NOTE: there is no any non linearity problem in the just reported mode of calculations. The extrapolated peak integrals are free from detector signal effects. The longer the circle line, the larger the calculated total integral value. The situation changes however when we move from the preliminary quantification to the final procedure: the translation of a detector signal into the substance weight - see the next part of this chapter below.
Also important: Because the position values of Pr are relative values always between zero and 1.00 there is no influence of the true mobile phase front position.
NEXT: The extrapolation procedure is valid also in multi phase programmed multi sample chromatograms which will have many differing mobile phase fronts from run to run.
NOTE: the sample substance amount does NOT change from run to run except when chemical material is damaged by light, air, or peroxides in dirty mobile phases.
See the table and picture below in this chapter.
From the area integral to the weight of a substance and to the relative weight concentration in a substance mixture
In order to end up quantitative analyses in error free correct weight-percent results we need substance specific correction factors and the signal linearization by polynomial calibration lines.
PLC integrals fundamentally correlate with the absolute substance weight on the plate non linear. As µ-PLC offers such sharp comparabilty standard deviation values we can much more critically check the non linearity between a PLC signal and the corresponding absolute sample weight. It is non linear from the very beginning at least under UV.
It is necessary to underline this NON LINEARITY remark and the one given above about perfect linearity for extrapolating integral values in circular chromatography: the latter is done to correctly compare small circle or bow data with large circle or bow data. The extrapolation puts the quantitative multi integration values onto the basis of absolute weight values.
THIS HOWEVER IS correct finally only, if we change a PLC integral into a substance specific value using substance specific correction factors. Even the peak areas measured with the quite linear flame ionization detector in capillary gas chromatography need substance specific correction factors in order to really reach error free information about benzene in gasoline or pesticides on apples. This remains true also by the use of quantitative inner standards in PLC quantitation. We need highly clean calibration substances or true values about their purity and substance specific correction factors based on non linearity corrections using polynomial interpolation. Only now we can get rid of systematic errors at the sampling step, and only after this analytical stress we know how clean a pharma top substance really is plus minus 0.05 % standard deviation or well: as PLC analysts still in 2010 accepted a +- 5% repeatability level in quantitative analyses: we may then reach a +- 15 % level of uncertainty analysis regardless of correct sampling and accurate integration based on only two repeated analyses.
The necessity to use substance specific correction factors in chromatography has been seen already about 50 years ago and was published in the first book on quantitation in gas chromatography by this author. Quite something could be improved up to now. Now we reach a +- 0.002 % relative standard deviation level in series quantitation of our most important energy substance methane. Comparing this level with the one given above may show what we could do in the future and in case analytical precision and accuracy is important (and wanted !). Health and security depends on error free data. Not always realized: The size of possible systematic errors can never be detected sharper than by the size of the repeatability standard deviation times 3 - roughly.
|