|
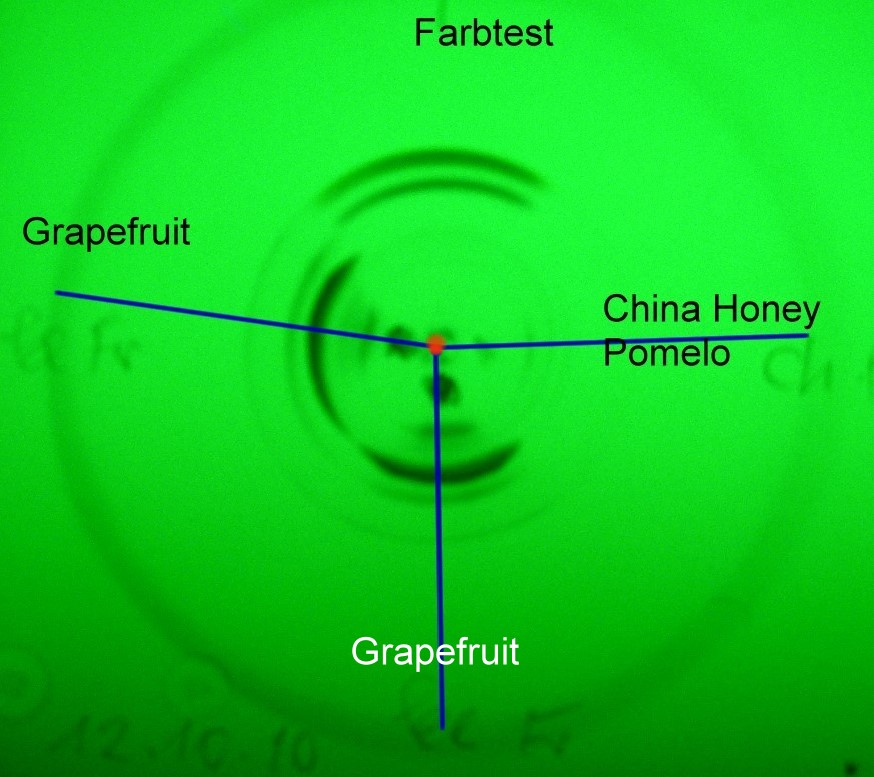
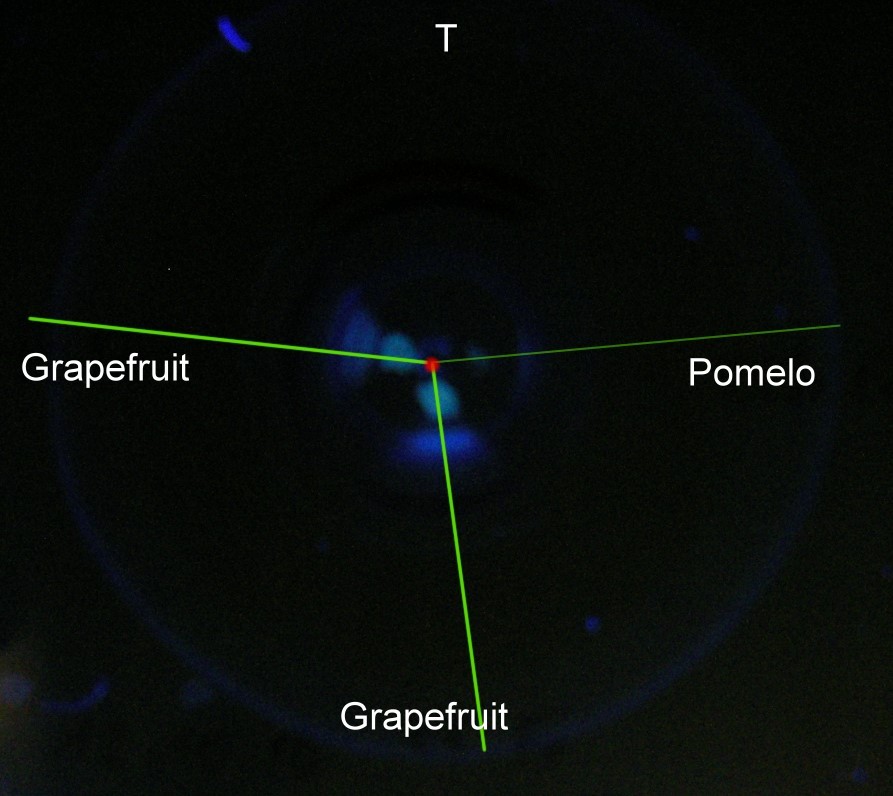
all figures |
|||||||||||||||||||||||
|
|
|||||||||||||||||||||||
|
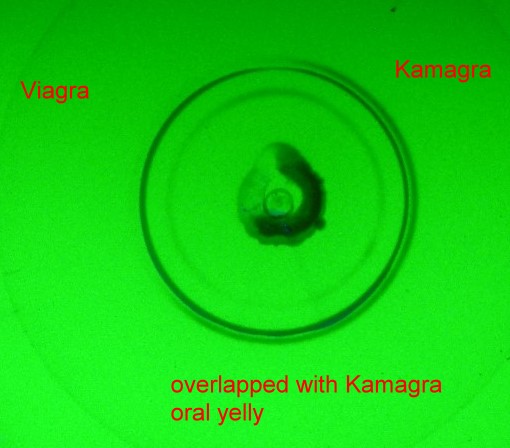
Multi focussing of critical samples Some samples are highly complex with respect of composition, solubility, homogeniety etc. One special example was the compare analysis of original Viagra and a product, which was told to “be equal to Viagra” , named Kamagra Oral Jelly containing 5 mg Sildenafil Citrate equivalent to Sildenafil 100 mg”. The Oral Jelly is deeply red colored and adsorbs on silicagel extremely strong. The compare analysis showed only after the ‘multi focussing effect’ discussed here Sildenafil and a by product in a nearly equal concentration as Sildenafil itself. Because of the strongly sorbing jelly red color we had to use several focussing steps after each other, starting with methanole, continued with ethanole, sec. propanole/water (70/30) and finally methylene chlorid always with drying steps in between. Now the original Viagra and the Kamagra sample could be compared chromatographically correct and showed no more problems in the overlapped zone. Well seen in the figure below it is of no question that Viagra is not Kamagra, but the latter contains Sildenafil plus an unknown substance of an equal amount as the Sildenafil itself - according to multi integration not shown here. This is the second circle seen right in the figure below at the Kamagra and the overlapped position. A very strong improvement of the separation power of the used standard silicagel glass plate of Merck (TLC Silica gel 60 F254 [1.05729.0001]) could be reached resulting in a formely never possible separation number of 50 instead of 10...15 as usual. This shows basically how important is focussing in PLC - not only in the circular mode. However only in this mode multi focussing is practically applicable. A next fact becomes understandable: Samples can have such a composition, that main products are locally nearly chemisorbed by special substances at the sampling position on / in the stationary phase, which would falsify a normal standard separation quantitatively. Many tailing effects could be overcome by specific multi focussing with solvents or gases, which allow preseparation from the position of primary application to a sharply focussed start circle. In case of the Kamagra sample it was partially ethanole but mainly the aquatic secundary propanole which finally allowed a correct compare chromatogram. Of course: WATER is not so easyly removed from silicagel. Thus a complete (room temperature) drying period by the on-plate-drying gas flow as used in µPLC for at least 15, better more than 15 minutes is a must . See the compare chromatogram below: |
 |
|
Theoretical details: Rf values and the Pr data in µ-PLC. Rf = s / f See for this and the following formulas figures 31 to 33 HERE. Position data into Rf (circular) values: Rf(circ) = (Ps - Po) / ( 1 - Po) Rf (linear) values are always larger than Rf(circular) values, as Rf (lin) = (Rf (circ))2 therefore [(Ps-Po) / (1-Po)]2 = Rf (lin) Application for a HPLC / PLC comparison in critical analytical conditions ka (in HPLC) / kb (in HPLC ) = ka (in PLC) / kb (in PLC). ka (in HPLC ) = ka (in PLC) * (kb (in HPLC) / kb (in PLC)) Thus we need two substances, which have differing k values and chemically equal chromatography systems. HERE is the reason for such theoretical discussions: H.Jork, W.Funk, W.Fischer, H.Wimmer: A huge amount of data about specific PLC reactions for identification and improved detection is found through CAMAGs literature service CBS, see CCBS in this Internet book.
|
| [Home] [Introduction] [Contents] [µ-PLC pictograms] [Multi Integration] [µ-PLC helps HPTLC] [Main errors in PLC] [Trace anal. by µ-PLC] [TLC HPTLC pictogr.] [Making a µ-PLC instr.] [PLC literature] [Details] [sel. Summary] [Balaton Papers] [Basel-Paper-2011] [3-Phases-Chrom] |